18 YEARS EXPERIENCE
We are a professional supplier of a variety of gas chemical products to meet your various needs.
Products Description
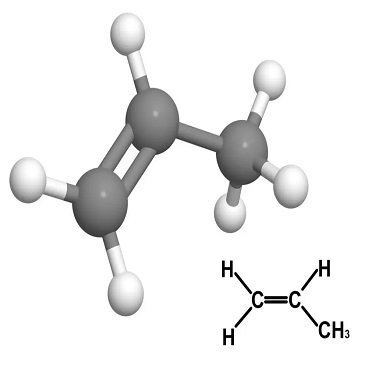
Name:Propylene
CAS :115-07-1
Molecular formula:C3H6
Molecular weight:42.08
EINECS:204-062-1
Physical properties
Propylene is also known as methylethylene and Propene. It is a colorless, flammable gas with a sweet taste. Its melting point is -185.2℃ and boiling point is -47.4℃. Its relative density in liquid state is 0.5193. It is easy to liquefy, with a critical temperature of 92℃ and a critical pressure of 4.56MPa. It forms an explosive mixture with air, with an explosion limit of 2.0% to 11.0% (volume). It is insoluble in water and soluble in organic solvents. High concentrations of propylene have an anesthetic effect on humans, and low concentrations can irritate the eyes and skin.
Propylene Properties
| Melting point | −185 °C(lit.) |
| Boiling Point | −47.7 °C(lit.) |
| Density | 1.49 |
| Vapor density | 1.48 (vs air) |
| Vapor Pressure | 15.4 atm ( 37.7 °C) |
| Refractive Index | 1.3567 |
| Flash point | -108 °C |
| Form | Colorless gas |
| Acidity coefficient (pKa) | 43(at 25℃) |
| Explosive limit | 11.1% |
| Odor Threshold | 13ppm |
| Water solubility | 0.33g/L(25 ºC) |
| Freezing point | -185.25℃ |
| Merck | 13,7941 |
| BRN | 1696878 |
| Dielectric constant | 1.9(20℃) |
| Stability | Stable. Highly flammable. Can easily form explosive mixture with air. Incompatible with strong oxidants, strong acids, halogens. |
Chemical properties
Propylene, chemical formula CH2=CHCH3. Molecular weight 42.08. It is a colorless flammable gas at room temperature and pressure. It has a slight odor unique to hydrocarbons. It is soluble in ethanol and ether, and slightly soluble in water. It has active chemical properties and can undergo oxidation, catalytic hydrogenation, addition, polymerization and other reactions. High-purity propylene is made from isopropanol by catalytic dehydration. Industrial products are obtained from petroleum pyrolysis gas. It is mainly used to make isopropanol, acetone, synthetic glycerin, synthetic resin, synthetic rubber, plastics and synthetic fibers.
Uses
Propylene is an important chemical raw material. Acrolein obtained by gas phase oxidation of propylene is used to produce acrylic acid, allyl alcohol, glyceraldehyde, hydroxyacetaldehyde and methionine, an important food and feed additive; acrylonitrile obtained by ammoxidation of propylene is an important raw material for synthetic fibers, synthetic rubber and plastics; allyl chloride obtained by chlorination of propylene can be further synthesized into allyl alcohol, propylene dichloropropanol, chloropropionitrile, etc., which are used to produce glycerol, epoxy resin, chlorohydrin rubber, surfactants, etc.; propylene alkylation to obtain isopropylbenzene, which is currently the main intermediate of phenol, and acetone is co-produced in the production of phenol; propylene is synthesized by carbonylation to obtain n-butyraldehyde and isobutyraldehyde, which can be derived from many organic synthesis intermediates, used in plasticizers, dyes, solvents, pesticides, etc.; propylene hydration to obtain isopropyl alcohol, which is used to produce acetone, isopropylamine and isopropyl esters; propylene dimerization to obtain ethylene, trimerization to obtain benzene, polymerization to obtain polypropylene, and propylene tetramerization to obtain dodecene, which is an intermediate of surfactants.
Production method
1. The C2 and C4 fractions are removed from the catalytic cracking gas of the refinery by distillation to obtain propylene and propane fractions, which are then distilled to obtain propylene.
2. The product of high-temperature cracking of petroleum hydrocarbons is a co-product of ethylene production.
3. Propane dehydrogenation. The catalyst is chromium oxide-aluminum oxide, the reaction temperature is 635℃, the propane conversion rate is 54%, the propylene selectivity is 76%, and the recovery rate is 93% (molecular ratio).
OUR ADVANTAGE
 18 YEARS EXPORT
18 YEARS EXPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES OEM/ODM SUPPORT
OEM/ODM SUPPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES OEM/ODM SUPPORT
OEM/ODM SUPPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES