18 YEARS EXPERIENCE
We are a professional supplier of a variety of gas chemical products to meet your various needs.
Products Description
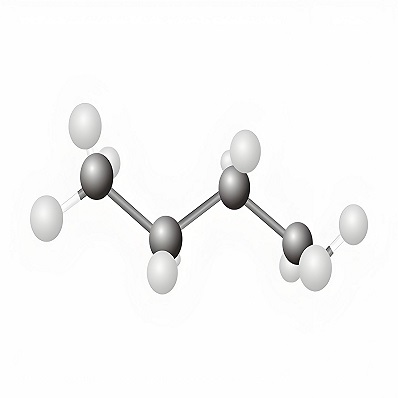
Product Name: n-Butane
CAS :106-97-8
Molecular formula:C4H10
Molecular weight:58.12
EINECS:203-448-7
Gaseous alkanes
N-butane is abbreviated as butane, with the molecular formula C4H10. A gaseous alkane that exists in petroleum and natural gas. There are two isomers, straight-chain n-butane and branched-chain isobutane. Both are colorless flammable gases at room temperature and pressure. N-butane has a slightly unpleasant odor. Relative density 0.6012 (0℃), 0.5788. Melting point -138.4℃. Boiling point -0.5℃. Critical temperature 152.01℃. Critical pressure 3.797MPa. Flash point -60℃. Refractive index 1.3259 (liquid, at saturated pressure), 1.0013 (gas, at normal pressure). Soluble in ethanol (18ml/1ml at 17℃ and 10.332×103Pa), ether (25ml/1ml), chloroform (30ml/1ml), slightly soluble in water (0.5ml/1ml). It forms an explosive mixture with air, with an explosion limit of 1.6% to 8.5% (volume fraction). Butane undergoes dehydrogenation in the presence of a catalyst to produce butene (1-butene and 2-butene) or butadiene, and isomerizes to isobutane in the presence of sulfuric acid or anhydrous hydrofluoric acid. Isobutane undergoes catalytic dehydrogenation to produce isobutylene (CH3)2C=CH2; isobutane can be used as an alkylating agent to react with olefins to produce branched alkanes with good anti-explosion properties, for example, it reacts with isobutylene to produce isooctane (see octane, octane number). In the oil refining industry, a large amount of n-butane is produced when gasoline is produced by cracking. It is liquefied with propane and a small amount of ethane and used as a fuel, namely liquefied petroleum gas, and is also used as a solvent and chemical raw material.
n-Butane Properties
| Melting point | −138 °C(lit.) |
| Boiling Point | −0.5 °C(lit.) |
| Density | 0.579 g/mL at 20 °C(lit.) |
| Vapor density | 2.11 (vs air) |
| Vapor Pressure | 3.21, 1.26, and 0.66 mM at 4, 25, and 50 °C, respectively (Kresheck et al., 1965) |
| Refractive Index | 1.3326 |
| Flash point | 45 |
| Form | gas |
| Odor | Faint unpleasant odor |
| Odor Threshold | 1200ppm |
| Water solubility | 73.24mg/L(25 ºC) |
| Merck | 1515 |
| BRN | 969129 |
| Henry's Law Constant | (atm?m3/mol): 0.356 at 5 °C, 0.454 at 10 °C, 0.568 at 15 °C, 0.695 at 20 °C, 0.835 at 25 °C (Ben-Naim et al., 1973) |
| Exposure Limits | TLV-TWA 800 ppm (~1920 mg/m3) (ACGIH), 500 ppm (1200 mg/m3) (MSHA). |
| Dielectric constant | 1.4(-1℃) |
| Stability | Stable. Highly flammable. Can easily form explosive mixture with air. Note low flash point. Incompatible with strong oxidants, strong acids and strong bases. |
Uses
1. It can be dehydrogenated to produce butadiene, oxidized to produce acetic acid and maleic anhydride, and can also react with sulfur in the gas phase to produce thiophene, etc.
2. It is mainly used as a standard gas for analytical and testing instruments in petrochemical enterprises
3. In addition to being used directly as a fuel, n-butane is also used as a solvent, refrigerant and raw material for organic synthesis. Butane is dehydrogenated to produce butene or butadiene in the presence of a catalyst, and is isomerized to form isobutane in the presence of sulfuric acid or anhydrous hydrofluoric acid. Isobutane is catalytically dehydrogenated to produce isobutylene. Isobutane can be used as a hydrocarbonizing agent to react with olefins to produce branched hydrocarbons with good anti-explosion properties. Butane can be catalytically oxidized to produce maleic anhydride, acetic acid, acetaldehyde, etc.; it can be halogenated to produce halogenated butane; it can be nitrated to produce nitrobutane; it can be catalytically catalyzed to produce carbon disulfide at high temperature; it can be converted into hydrogen by steam conversion. In addition, butane can be used as a motor fuel blend to control volatile components; it can also be used as a deasphalting agent for heavy oil refining; a wax precipitant in oil wells; an overflow agent for secondary oil recovery, a resin foaming agent, a refrigerant for converting seawater into fresh water, and an olefin agent Geller polymerization solvent.
Production method: Oilfield gas, wet natural gas and cracked gas all contain n-butane, which can be obtained by separation.
(1) Separate oilfield gas and wet natural gas by pressurizing and condensing them to obtain liquefied petroleum gas containing propane and butane, and then separate butane by distillation.
(2) Separate the C4 fraction from petroleum cracking. The gas obtained by normal temperature and vacuum distillation in the refinery can obtain a large amount of C4 fraction through reforming, catalytic cracking, coking, thermal cracking, and hydrogenation cracking. The C4 fraction obtained by reforming, hydrogenation and atmospheric and vacuum distillation is mainly butane (n-butane and isobutane). The C4 fraction produced as a by-product of the ethylene plant also contains butane. For example, the yield of butane in the medium-depth cracking product of naphtha is 0.19% (weight), accounting for 6.5% of the C4 fraction. The tail gas from the catalytic cracking unit is fractionated to separate the C3 fraction, isobutylene and C5 fraction, and then sent to the front acetonitrile extraction distillation tower from the bottom of the tower to obtain more than 90% of n-butane from the top of the tower.
OUR ADVANTAGE
 18 YEARS EXPORT
18 YEARS EXPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES OEM/ODM SUPPORT
OEM/ODM SUPPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES OEM/ODM SUPPORT
OEM/ODM SUPPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES