18 YEARS EXPERIENCE
We are a professional supplier of a variety of gas chemical products to meet your various needs.
Products Description
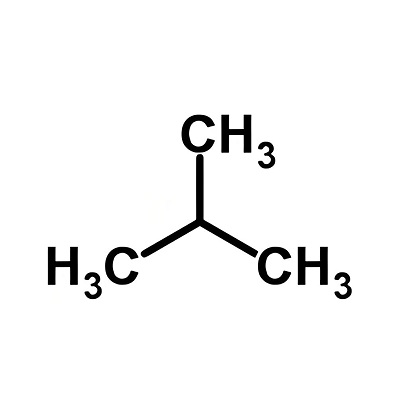
Product Name: Isobutane
CAS:75-28-5
Molecular formula:C4H10
Molecular weight:58.12
EINECS:200-857-2
Introduction
Isobutane is a colorless, flammable gas that is slightly soluble in water and stable in nature. It can form explosive mixtures with air, with an explosion limit of 1.9 to 8.4% (volume). It exists in petroleum and natural gas. It is industrially produced by cracking petroleum, and can also be obtained by isomerizing n-butane. Isobutane is mainly used to prepare isooctane as an improver for gasoline octane number; it is also used as a refrigerant.
Isobutane is a hydrocarbon refrigerant, a colorless flammable gas. Its specific gravity is 0.5510 (25°C), its melting point is -145°C, and its boiling point is -11.73°C. It exists in petroleum gas, natural gas, and cracked gas. Isobutane can also be generated by isomerization of n-butane. It is slightly soluble in water, has stable chemical properties, and forms an explosive mixture with air, with an explosion limit of 1.9% to 8.4% (volume).
Isobutane Chemical Properties
| Melting point | −160 °C(lit.) |
| Boiling Point | −12 °C(lit.) |
| Density | 2.064 g/mL at 25 °C(lit.) |
| Vapor density | 2.01 (21 °C, vs air) |
| Vapor Pressure | 72.2 psi ( 37.7 °C) |
| Refractive Index | 1.3518 |
| Flash point | -83 °C |
| Form | Colorless gas |
| Color | Colorless |
| Explosive limit | 8.3% |
| Water solubility | 48.9 mg/kg at 25 °C (shake flask-GC, McAuliffe, 1963, 1966) |
| BRN | 1730720 |
| Henry's Law Constant | 1.171 atm?m3/mol at 25 °C (Hine and Mookerjee, 1975) |
| Exposure Limits | NIOSH REL: TWA 800 ppm (1,900 mg/m3). |
| Stability | Stable. Highly flammable. May form explosive mixture with air. |
Chemical Properties
Colorless, Odorless, Flammable and Explosive Gas, Boiling Point About -11℃. Vapor Pressure About 0.39mpa at 21℃.
Chemical Applications There Are Four Main Ways to Utilize Isobutane in the Chemical Industry:
(1) Alkylation for the Production of Alkylated Gasoline; Dehydrogenation to Produce Isobutylene;
(2) Steam Cracking to Produce Ethylene, Propylene, Etc.;
(3) Isobutane and Propylene Co-Oxidation to Produce Propylene Oxide and Tert-Butyl Alcohol;
(4) Isobutane and Formaldehyde to Produce Butadiene (This Technology Has Been Industrialized in Russia).
In Addition to Being Directly Used as a Civilian Fuel, Most of Isobutane Is Used for Alkylation to Produce Automotive Fuel Oil Blending Components, And Other Chemical Utilization Methods Are Rarely Used. The Co-Oxidation of Isobutane and Propylene to Produce Propylene Oxide and Tert-Butyl Alcohol Has the Advantage of No Environmental Pollution, But the Investment Is Relatively High. The Domestic Production of Propylene Oxide Generally Adopts the Chlorohydrin Method, Which Is Extremely Polluting to the Environment, But Its Advantage Is That the Investment Is Relatively Small.
Uses
1. Isobutane is mainly used to produce isooctane with isobutylene through alkylation, and is used as a gasoline octane improver. It can produce isobutylene and propylene through cracking. It can produce alkylated gasoline through alkylation with isobutylene and propylene. It can produce methacrylic acid, acetone and methanol, etc. It can also be used as a refrigerant, aerosol and inflator.
2. Mainly used as a standard gas for analysis and testing instruments in petrochemical enterprises
Production method
Isobutane exists naturally in petroleum gas, natural gas and cracked gas. It is obtained by separating the carbon four fraction produced in the process of petroleum cracking. Or it is obtained by adsorption or freezing of natural gas after fractionation with surfactant.
OUR ADVANTAGE
 18 YEARS EXPORT
18 YEARS EXPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES OEM/ODM SUPPORT
OEM/ODM SUPPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES OEM/ODM SUPPORT
OEM/ODM SUPPORT QUALITY CERTIFICATES
QUALITY CERTIFICATES